1D Lithium-Ion Battery Model for the Capacity Fade Tutorial
Application ID: 12667
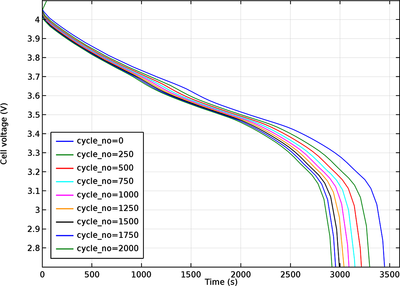
Side reactions and degradation processes may lead to a number of undesirable effects, causing capacity loss in lithium-ion batteries. Typically, aging occurs due to multiple complex phenomena and reactions that occur simultaneously at different places in the battery, and the degradation rate varies between certain stages during a load cycle, depending on potential, local concentration, temperature, and the direction of the current. Different cell materials age differently, and the combination of different materials may result in further accelerated aging due to, for instance, “crosstalk” electrode materials.
This tutorial demonstrates how to model aging in the negative graphite electrode in a lithium-ion battery, where a parasitic solid-electrolyte-interface (SEI) forming reaction results in an irreversible loss of cycleable lithium. The model also includes the effect of increasing potential losses due to the resistance of the growing SEI film on the electrode particles, as well as the effect of a reduced electrolyte volume fraction on the electrolyte charge transport.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.
