Control-Release Anesthetics to Enable an Integrated Anesthetic-MSC Therapeutic
Introduction: While general anesthetics control pain via consciousness regulation, local anesthetics (LAs) act by decreasing sensation in the area of administration by blocking nerve transmission to pain centers. Perioperative intra-articular administration of LAs is a commonly employed procedure in orthopedic procedures to minimize patient surgical and post-surgical pain. LAs are also co-administered with cellular mesenchymal stromal cell (MSC) therapies for a variety of tissue regenerative and inflammatory applications; however, our previous work determined that LA can decrease MSC immunomodulatory and anti-inflammatory function[1]. Therefore, finding an improved method to co-administer LAs with cells has become critically important.
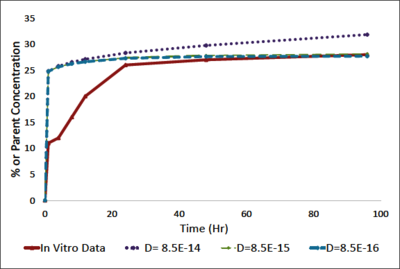
Material and Methods: In order to develop a sustained release LA system, hydrogenated soy phosphatidylcholine unilameller liposomes containing bupivacaine were encapsulated in a 2.2wt% alginate hydrogel. Molecular dynamics characterized the liposomal-bupivacaine formulation. Bupivacaine release from liposomes was determined using HPLC. The COMSOL Multiphysics® software was used to model the fluid dynamics of bupivacaine release out of the alginate-liposome formulation. An incompressible Navier-Stokes with diffusion-convection model was used with a preconditioned iterative solver using a time stepping method. The model was isothermal with a no flux condition except for the surface between the alginate and the media. The diffusivity of bupivacaine in the media was 1E-10 mol/m3 and the diffusivity in the alginate-liposome formulation was determined experimentally to be 8.5E-15 mol/m3.
Results and Discussion: We have developed a sustained release LA delivery model that could enable the co-administration of LAs and MSCs. Our molecular dynamic results indicated that the lipoprotein would form stable vesicular structures around the bupivacaine. The retention time of bupivacaine in liposomes was found to be approximately 4.7 min in a column, with release for up to 24hours. Encapsulation of liposomes within an alginate matrix leads to sustained release of bupivacaine as compared to bupivacaine-containing liposomes alone. Furthermore, drug release was maintained for a minimum of 4 days. Modeling using the COMSOL® software showed that the release of drug from the liposome-alginate formulation decreased the cell apparent dose. An initial bupivacaine concentration of 1mM yielded a cell apparent dose of less than 0.1mM, which was previously determined as a threshold to ensures 90% cell viability[1].
Conclusions: The significance in our work is in providing multi-day pain management in post-operative procedures with the co-administration of LA and cells, such as MSCs. Our current studies indicate that bupivacaine encapsulated in our liposome-alginate formulation yields a sustained release of bupivacaine over 4 days, which is longer than current available formulations. Moreover, it decreases the cell apparent dose of bupivacaine to ensure MSC viability. The model built with the COMSOL® software allows for modifications to multiple parameters as once, such as concentration, alginate/liposome parameters, and geometry of the system, to ensure optimal experimental design and scale-up. Future studies should focus on in vitro testing of the liposome-alginate formulation with MSCs to validate the model, as well as characterization of cell secretions to understand MSC function in the presence of this sustained release bupivacaine system.
Download
- davis_poster.pdf - 1.21MB
- davis_abstract.pdf - 0.02MB
